New protein regulation mechanism identified
26/11/2018
The control of the lifetime of a cell protein is an essential mechanism necessary to regulate its activity and help the cell cycle function correctly. This cycle permits cells to divide and make new tissue grow and regenerate. The control of different cell cycle stages is conducted by different types of proteins which substitute one another as the cycle advances. The cells have designed a mechanism to tag the proteins which must be degraded, a process known as ubiquitination, and the tag consists of forming a chain with the ubiquitin protein. The proteins tagged with ubiquitin are eliminated through a what is known as proteosomes, protein complexes in charge of degrading them. The regulation of this process is very important in making the cell cycle function correctly, given that alteration in cell proliferation could lead to cancerous processes in which the cells would grow uncontrollably.
In the research recently published in Nature Communications, researchers present the structural characterisation and discovery of a new regulation mechanism of an enzyme which severs the ubiquitin chains, known as USP25. This enzyme eliminates the ubiquitin chain from the tagged proteins and has a huge effect on cell function since it prevents them from being degraded by proteosomes.
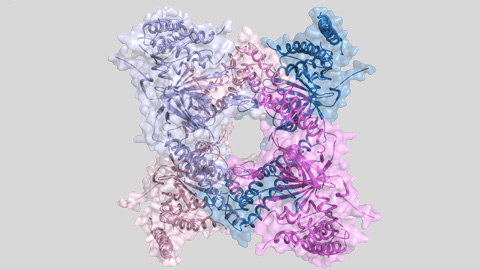
The enzyme's three-dimensional structure, obtained thanks to protein crystallography and the ALBA synchrotron light, has allowed researchers to observe the protein assembly with two different types of quaternary structures: tetramer and dimer. And what is more important is that the tetramer is inactive whereas the dimer is active. Therefore, the alternation of these two assemblies regulates the enzyme's activity and, at the same time, the lifetime of important cell proteins.
The study was conducted at the laboratory of Dr David Reverter, who directs the protein structures group at the Institute of Biotechnology and Biomedicine at the UAB (IBB). Also collaborating were the group led by Dr Virginia Amador from the biomedical research centre IDIBAPS at the Hospital Clínic. The lead author of the study was Dr Bing Liu, also with the IBB and the UAB Department of Biochemistry and Molecular Biology.
Original article:
Liu, B., Surena-Gomez, M., Zhen, Y., Amador, V., Reverter, D. A quaternary tetramer assembly inhibits the deubiquitinating activity of USP25. Nature Communications (2018). DOI: 10.1038/s41467-018-07510-5.