The COX-2 dimer as a new therapeutic target against cancer
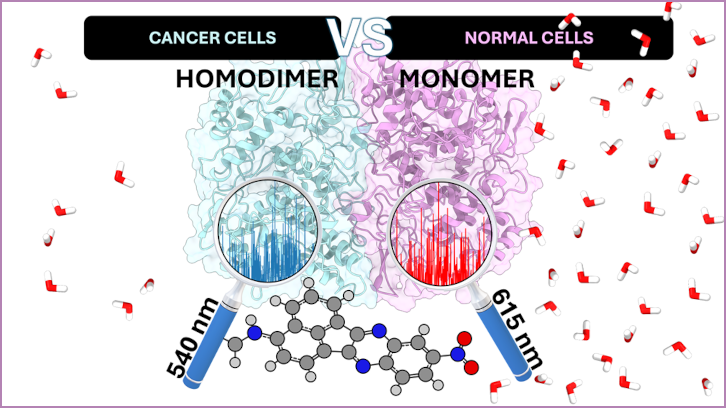
The MolBioMed research group of the Department of Chemistry, in collaboration with the University of Barcelona, has published a study that demonstrates the direct relationship between the COX-2 dimer and cancer. The computational study shows the different behavior of the fluorescence spectrum of the NANQ-IMC6 compound when interacting with the monomer (present in inflamed tissues) and with the dimer (present in cancer cells).
Inflammation is a key defense mechanism against injury and pathogens and becomes detrimental when chronic, as it promotes tissue destruction, cancer progression, and immune evasion. Proinflammatory mediators derived from arachidonic acid, such as prostaglandins and leukotrienes, produced by the enzymes cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX), drive acute inflammation, with COX-2 prostaglandin E2 playing a crucial role. Although COX-2 inhibitor drugs, such as the well-known NSAIDs, show potential in cancer prevention, their non-selectivity or prolonged use causes serious side effects, which limits their use.
Interestingly, COX-2 is overexpressed in cancerous tissues and acts as a cancer marker, allowing the use of fluorogenic compounds such as NANQ-IMC6, which selectively target COX-2 and show different fluorescence in cancerous and inflammatory tissues. The hypothesis of this work is that the different response is due to COX-2 dimerization (the union of two units of the same enzyme) in tumors, opening new strategies for the design of selective drugs that mitigate COX-2-induced cancer growth without adverse effects. This study uses advanced simulation techniques to explore the molecular basis of dimerization-induced fluorescence changes, shedding light on new specific therapeutic avenues against cancer.
The study focuses on the theoretical description of the fluorescence spectrum of the NANQ-IMC6 ligand in the monomeric (one enzyme unit) and homodimeric (two units with the same function) isoforms of the human COX-2 enzyme using molecular docking, molecular dynamics, and QM/MM (Quantum Mechanics/Molecular Mechanics) simulations. The molecular dynamics simulations show a different behavior of the ligand in the three possible binding cavities of the enzyme, especially in cavity B of the COX-2 dimer, which is undoubtedly responsible for the behavior change in the fluorescence spectrum. Based on the structures of these molecular dynamics, QM/MM simulations have been performed in the S1 excited state of the monomer and the homodimer, with the NANQ unit included in the QM region. After these simulations, it has been possible to construct the theoretical fluorescence spectrum of the compound, which shows how the dimer has an energy shift of -75 nm with respect to the compound in the monomer. This value is comparable to those observed experimentally between cancerous and inflammatory tissues.
Furthermore, it has been observed that between the two monomeric units present in the dimer, there is an allosteric interaction that converts our system into a functional heterodimer. One of the monomers acts on the other in a way that allows a conformational change in the other cavity, which, as far as our study is concerned, prevents the ligand from positioning itself in the same way on both sides and modifies the photochemistry of our compound. Recent studies experimentally demonstrate that the behavior of each unit of the dimer is not always the same (hence the concept of heterodimer). Our results verify the possible modulatory involvement of one of the units in the formation of the dimer.
In conclusion, the results show that the overexpressed COX-2 in cancerous tissues exists as a dimer, while in inflammatory lesions it is presented as a monomer. This opens the door to designing new anti-inflammatory drugs that selectively inhibit the COX-2 homodimer to treat cancer with fewer side effects.
Department of Chemistry
MolBioMed (Molecular Biomedicine) Research Group
Universitat Autònoma de Barcelona
References
Pérez-Sánchez, Á.; Curutchet, C.; González-Lafont, À.; José M. Lluch. First-principles simulations of the fluorescence modulation of a COX-2 specific fluorogenic probe upon protein dimerization for cancer discrimination. Protein Science 2025, 43 (1), e70001. DOI: http://dx.doi.org/10.1002/pro.70001

