Production of Toxin-Free Bacterial Amyloid
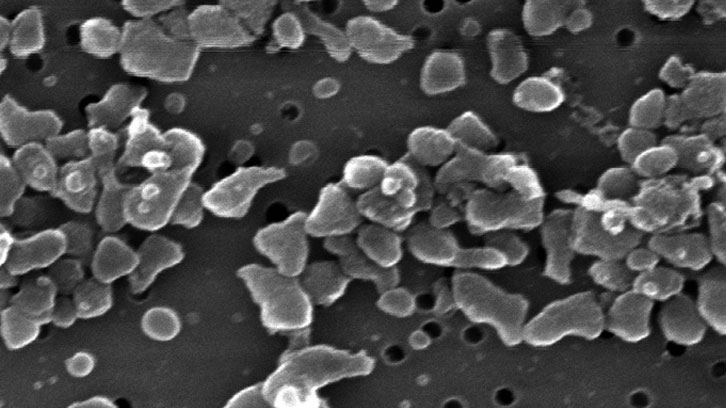
Bacteria inclusion bodies are cost-effective nanostructured protein materials produced in bacteria with intriguing biomedical applications, including protein drug release and biofunctional topographies.
Inclusion bodies belong to the newly identified class of non-toxic, functional amyloids that acting as biomimetics of secretory glands in the endocrine system, combine release of functional protein with mechanical stability. However, the uses of inclusion bodies might be largely narrowed by the presence of residual endotoxins from the producing bacterial cells.
In collaboration with Prof Uwe Mamat, from the Leibniz-Center for Medicine and Biosciences (Borstel, Germany), who has developed several Escherichia coli strains deficient in the cell wall lipopolysaccharide, we have produced and characterized functional inclusion bodies in K-12 derivative endotoxin-free strains.
Our data indicate that these materials, being indeed free from potentially hazardous cell contaminants keep both the mechanical and biological properties (including cell penetrability) that make them appealing for their use in biological interfaces. These data indicate, then, that Prof’s Mamat strains are suitable to be used as bacterial factories for the production of fully biocompaticle and highly tuneable protein materials of bacterial origin based on functional amyloids.
Fabián Rueda
Olivia Cano-Garrido
Joaquin Seras-Franzoso
Elena García-Fruitós
Antonio Villaverde
Institute of Biotechnology and Biomedicine (IBB)
References
Rueda, F.; Cano-Garrido, O.; Mamat, U.; Wilke, K.; Seras-Franzoso, J.; García-Fruitós, E.; Villaverde, A. Production of functional inclusion bodies in endotoxin-free Escherichia coli. Applied Microbiology and Technology. 2014, vol. 98, num. 22, p. 9229-9238. doi: 10.1007/s00253-014-6008-9.

