Dimers and polymer chains: effects of functional group position in a heterocyclic ligand
Synthesizing more efficient materials with a diversity of properties is a reality thanks to the possibility of manipulating the molecular geometry and structure of compounds. In this article, the Design of Metal Organic Materials group at the UAB presents six compounds using metallic cations Zn(II), Cd(II) and Hg(II), and two flexible ligands. These ligands, which are also hybrid, have the same functional groups, but are coordinated differently in space and have different forms depending on the para- and ortho- isomers used.
Crystal engineering is a field of chemistry dedicated to the design of coordination compounds with tuneable structures and geometries and is arguably one of the most active fields of research1.It is based on the combination of metallic cations, acting as nodes, and organic ligands binding them. By modifying these ligands, the final crystalline network can be altered2.
The search for more efficient materials possessing new functionalities has led to the emergence of flexible ligands (in opposition to rigid ligands which were favoured previously) including heterocyclic nitrogen donors. Moreover, the design of hybrid ligands, that is, ligands possessing different donor atoms, is also thriving3.
Schematic representation of the six resulting coordination compounds.
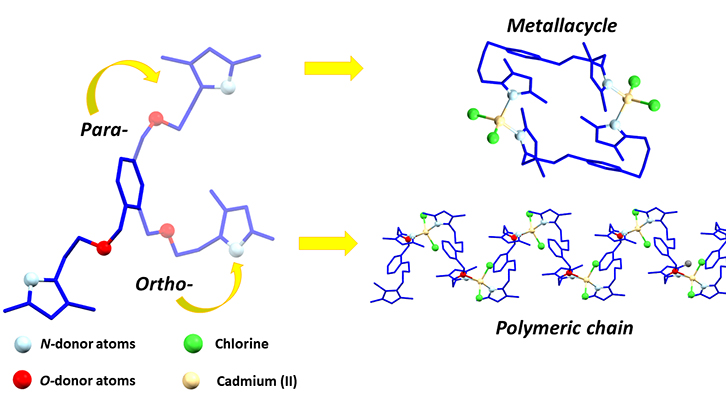
In this work, two flexible ligands possessing heterocyclic nitrogen donors (pyrazole) and oxygen atoms, thus being a N,O-hybrid donor, are presented. The two synthetized ligands have the same functional groups in their structure, but the relative position of its substituents is different. Therefore, the para- and the ortho- isomers are obtained. In this work, the effect of their different substituent position in the structure of the resulting coordination compounds has been studied. Selected metal cations were Zn(II), Cd(II) and Hg(II).
This study yielded six compounds, three for each ligand. All compounds have been fully characterized via analytical and spectroscopic techniques and their crystal structures elucidated using X-ray single crystal diffraction.
It has been observed that small changes in substituent position has a huge impact on the shape and properties of the resulting compounds. Those obtained with the para- isomer have a thirty-four membered dimeric ring structure. On the other hand, those including the ortho- isomer are infinite polymeric chains. Moreover, the coordination behavior of the ortho- isomer is different in compounds containing Zn(II) from those containing Cd(II) or Hg(II). In the Zn(II) compound the ligand is bonded via nitrogen atoms, whereas in Cd(II) and Hg(II) both nitrogen and oxygen atoms are bonded. This behaviour also affects the overall shape of the compounds; the Zn(II) one has a zigzag chain shape, whereas Cd(II) and Hg(II) ones have an helical chain shape.
Finally, the solution behaviour of these compounds was also studied via 1H NMR, 13C NMR, UV-Vis spectroscopy and photoluminescence measurements.
Inorganic Chemistry Area.
Department of Chemistry.
Universitat Autònoma de Barcelona (UAB).
References
J. Soldevila-Sanmartín, M. Guerrero, D. Choquesillo-Lazarte, J.G. Planas, J. Pons. Dimeric metallacycles and coordination polymers: Zn(II), Cd(II) and Hg(II) complexes of two positional isomers of a flexible N,O-bispyrazole derived ligand. Inorg. Chim. Acta 506 (2020) 119549. https://doi.org/10.1016/j.ica.2020.119549
Referències complementàries
[1] L. Brammer. Developments in inorganic crystal engineering. Chem. Soc. Rev. 33 (2004) 476–489. https://doi.org/10.1039/b313412c.
[2] W. Lu, Z. Wei, Z.Y. Gu, T.F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch, H.C. Zhou. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 43 (2014) 5561–5593. https://doi.org/10.1039/c4cs00003j.
[3] C. Sanchez, K.J. Shea, S. Kitagawa. Recent progress in hybrid materials science. Chem. Soc. Rev. 40 (2011) 471. https://doi.org/10.1039/c1cs90001c.

