Characterization of three compounds with Cu (II) carboxylate ligands
The study of chemistry in terms of the coordination of Copper (II) and Zinc (II) compounds with ligands containing carboxylate groups of great interest given the labile and versatile nature of the ligands, since these compounds are useful in fields such as catalysis, molecular electronics, magnetism and gas storage. In addition, the compounds may have different coordination modes, in this case, with Cu (II) it would be monodentate, chelate, bridge, etc., and Cu (II) ions may have different coordination numbers and geometries, from the tetrahedral to the octahedral form.
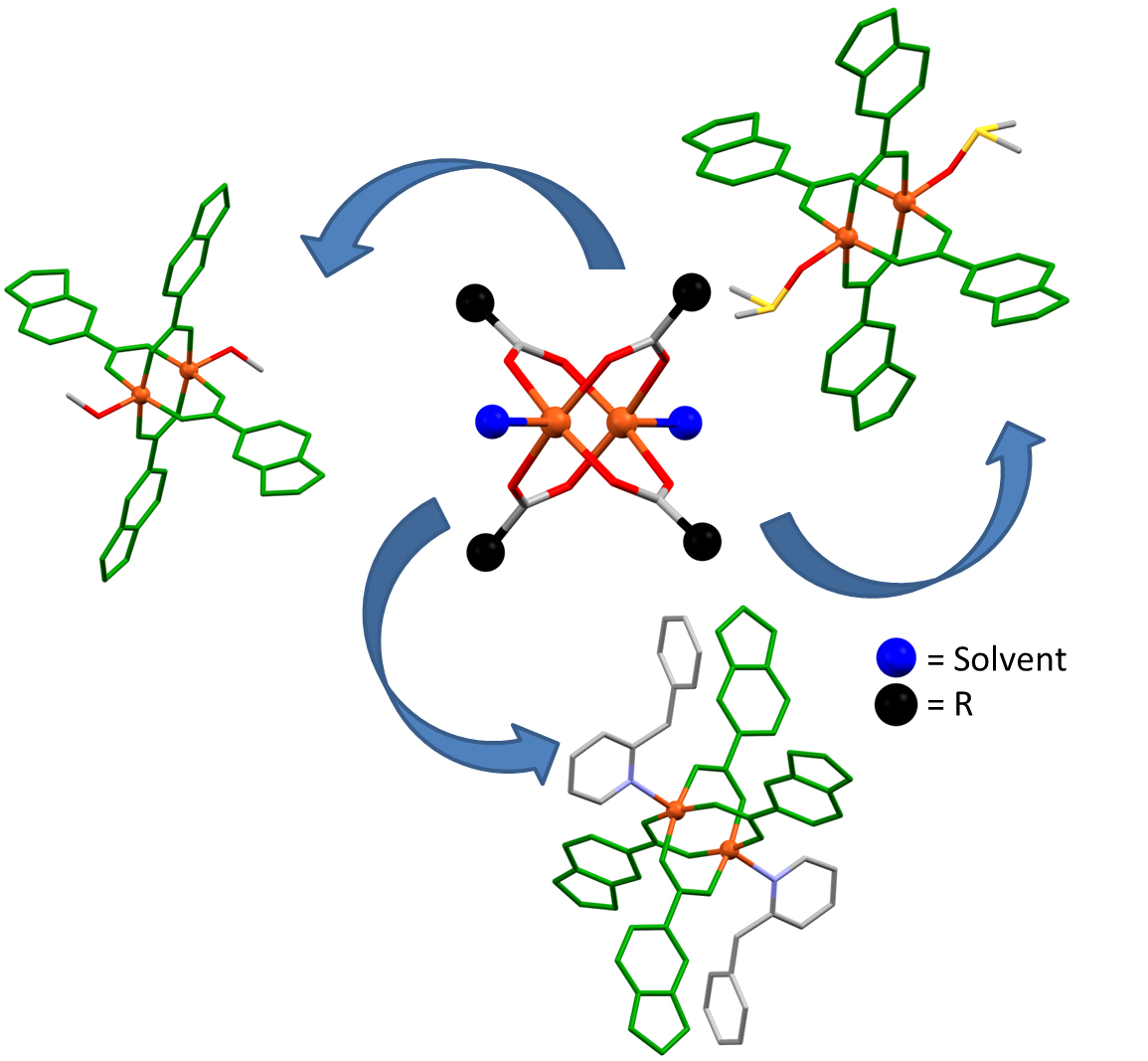
An important family of Cu (II) carboxylate compounds are those that have a "paddle-wheel" structure, due to the four ligands that form a bridge between two metals and the fact that one of the positions (apical) of the environment of one of the metals is occupied by another molecule and not by the paddle wheel.
Recently, the UAB's "Design of Metal Organic Materials" research group focused, on the one hand, on the preparation, synthesis and characterization of three compounds with a "paddle-wheel" structure of Cu (II), a metal with oxidation status +2. The drawback, however, is that, for these types of compounds, there is no standard method of synthesis, since they have a structure that makes them useful as starting products of other compounds, thanks to their core that remains unchanged after different reactions. For this reason, the study presents three different synthetic methods to obtain compounds with a "paddle-wheel" structure: analytical, spectroscopic and single crystal X-ray diffraction techniques. On the other hand, the research group studied the supramolecular structure of the compounds in order to analyze their potential for application in the catalysis, separation and storage of gases. Finally, the measurement of the thermal stability (TG / DTA) of compounds was recorded to indicate at what temperature the products are stable, that is, at what temperature they decompose and how this decomposition takes place.
The resolution of the crystalline structure by X-ray diffraction of the three compounds confirms that they all have four carboxylate ligands forming a bridge between the two metal atoms of Cu (II) and the analysis of the supramolecular structures of these demonstrates the formation of 1D and 2D networks.
Also interesting was the analysis and study of supramolecular structures formed by intermolecular interactions. Specifically, in the three mentioned compounds, the substitution of the apical positions by different auxiliary ligands leads to the construction of different intermolecular interactions, giving rise to new architectures.
References
“Synthesis and characterization of three new Cu(II) paddle-wheel compounds with 1,3-benzodioxole-5-carboxylic acid”. Francisco Sánchez-Férez, Joan Soldevila-Sanmartín, José A. Ayllón, Teresa Calvet, Mercè Font-Bardía, Josefina Pons, Polyhedron 2019, 164, 64-73.
J.M. Rueff, N. Masciocchi, P. Rabu, A. Sironi, A. Skoulios, Eur. J. Inorg. Chem. (2001) 2843.
N. Abdullah, Y. Al-Hakem, H. Samsudin, N.S.A. Tajidi, Asian J. Chem. 26 (2014) 987.
C.R. Groom, I.J. Bruno, M.P. Lightfoot, S.C. Ward, Acat Crystallogr. Sect- B Struct. Sci. Cryst. Eng. Mater. 72 (2016) 171.
M. Guerrero, S. Vázquez, J.A. Ayllón, T. Calvet, M. Font-Bardía, J. Pons, ChemistrySelect 2 (2017) 632.
F. Sánchez-Férez, M. Guerrero, J.A. Ayllón, T. Calvet, M. Font-Bardía, J. Giner Planas, J. Pons, Inorg. Chim. Acta 487 (2019) 295.

