Experimental procedures
Substance administration
In procedures that involve the administration of drugs or other substances, the following basic aspects must be considered:
Before carrying out the procedure, it will be necessary to consider the potential toxic or harmful effects that may occur as the result of administration. If, due to the information available, effects of this type can be predicted, either at a systemic or local level, it will be necessary to define specific supervision in the form of the corresponding procedure in order to detect the appearance of these effects and the potential corrective measures to be applied to the affected animals.
Enteral (oral):
-
Intake through food or drinking water. It is the simplest method, but it will be necessary to consider the capacity of the established design to determine the amount of substance ingested and whether or not this can influence the results (for example, in substances administered in drinking water in animals stabled in groups). This type of administration will not be possible for substances that irritate the gastric wall.
-
To promote the animal's willingness to comply with oral intake, it can be useful to make the substance to be ingested more attractive to the palate, for example by diluting it in gelatine, or sweetening the solution.
-
In some cases, it is advisable to use gastric tubes (0.8mm external diameter for mice and 1-2mm for rats) or a curved needle to provide a dose for small animals.
Parenteral <>
When an administration of this type is carried out, some basic aspects must be considered: the needles must be clean, pointed and sterile, of an appropriate size and thickness according to the animal, the route, the site of administration and the viscosity of the fluid must never exceed the maximum recommended volume or the bolus injection speed. To inject large volumes, the dose must be divided into several administrations in different sites. When the pH is too high (>8) or too low (<4.5) it must be diluted in saline solution to avoid irritation. The solution to be administered must be tempered (to room or body temperature) to avoid any pain produced by injecting cold substances. The skin must first be cleaned with a disinfectant solution (povidone-iodine or chlorhexidine) to avoid infections but not excessively, so as not to cause any irritation or the elimination of bacterial flora.
Recommendations for intracerebral administration are provided in the section on stereotactic techniques.
The main routes are:
-
Intradermal (I.D).
-
Subcutaneous (S.C): This is the simplest and most tolerated method. It allows relatively large volumes to be administered but the substances are absorbed relatively slowly.
-
Intramuscular (I.M): It is necessary to aspirate before injecting to check that no blood flows through the needle (if so, it will have to be injected at another site). It does not allow for a very large volume, so in many cases it will be necessary to inject it at different sites or try an alternative route.
-
Intraperitoneal (I.P): This is very common in rodents in which I.V or I.M access is difficult or inadequate. Care must be taken to avoid injuries derived from the injection (peritonitis, liver injury, etc.). In case of repeated administrations, the flank where the administration is carried out must be alternated for each puncture.
-
Intravenous (IV): The drugs have fast and powerful effects and allow large volumes and low doses to be injected, as well as the administration of substances with a pH or osmotic concentration that deviate from physiological ones. For repeat and proximate administrations, it will be necessary to fit a catheter, which must be monitored periodically to keep it in optimal condition.
Topical route and inhalation
-
In topical administration (usually used for a local site), the surface on which the substance is applied (a solution or cream) must be properly shaved. In some cases, the application of hair removal creams may be useful. Depending on the type of animal, appropriate physical restraint should be considered.
-
It will be necessary to monitor the potential irritation of the skin or mucous membranes.
-
The inhalation route requires masks or vaporisers adapted to each animal species to ensure correct dosage.
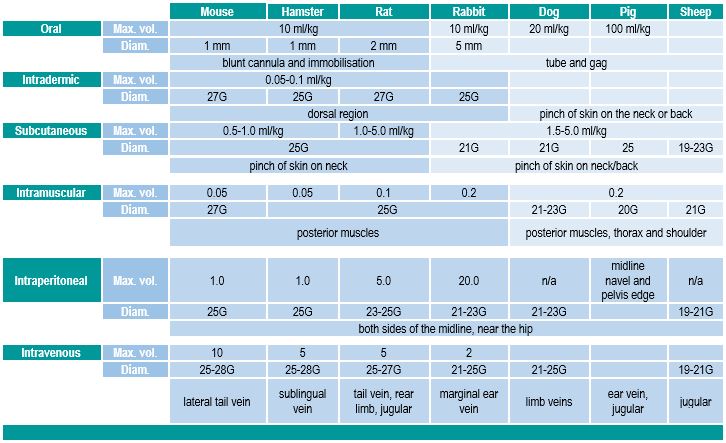
*depending on the administration route and the animal species
Blood extraction
The following link provides recommendations for procedures that involve blood extraction, as well as a table listing blood volumes and usual puncture sites according to species. Some of these general criteria are:
*You can find more information and useful videos here
In vitro procedures
In vitro procedures are those in which the only handling of the animal involves slaughtering them, using humanitarian euthanasia methods.
When carrying out such a procedure, the following considerations must be taken into account.
Tumours in rodents
All procedures in which neoplasms are experimentally induced, must define the endpoint criteria; a set of conditions under which the affected animals will be euthanised. Some of the general criteria are:
-
The fact that the weight of the tumour is greater than 10% of the animal's weight. Since in some cases, for example, depending on the shape of the tumour, it is difficult to calculate its weight, it will be necessary to define in the corresponding procedure form how the weight of the tumour will be monitored and what measures will be taken when these limits are exceeded.
-
The fact that the tumour ulcerates before reaching this weight.
-
In some studies, it may occur that, either due the tumour itself or due to the experimental design (for example, procedures that involve antitumor therapy) there may be a marked weight loss. In these cases, an endpoint criterion would be the loss of 15-20% weight or a 15-20% decrease in weight gain in animals during their growth period.
-
Some tumours can interfere with the proper function of certain vital organs, particularly those involved in correct nutrition (inability to move to get food or water, inability to swallow, etc.). In these cases, if it is not possible to correct it, the animals should be euthanised regardless of the weight of the tumour or the animal.
Animals with induced tumours should be monitored daily by the staff members participating in the procedure.
Euthanasia (agents and methods)
The primary criteria for euthanasia, in terms of welfare, are that the method is not painful, achieves rapid unconsciousness and death, requires minimal immobilisation, avoids anxiety, is appropriate for the age, species and health of the animal, minimises fear and stress for the animal, is reliable, reproducible, irreversible, simple to administer (if possible in low doses) and safe for staff members. As far as possible, it should be aesthetically acceptable to staff members.
Decree 214/1997 (Chapter 9, art.28c) indicates that it is the function of animal experimentation ethics committees to control the use of humanitarian euthanasia methods.
Recommendations for euthanasia in experimental animals.
Statistical analysis
Decree 214/1997 (Chapter 9, art.28a) indicates that it is the function of animal experimentation ethical committees to provide information about the possibility of reaching valid conclusions with the smallest possible number of animals.
The Appplied Statistics Service (SEA), which is a Technical Unit that forms part of the Scientific-Technical Services at the UAB, provides specialised consultancy in the field of data analysis.